Scapa Healthcare Accelerates the Development and Commercialization of a Postpartum Hydrogel- Dressing for a Leading Consumer Company
August 2023
Summary
- A leading pregnancy and postpartum product manufacturer wanted to develop a customized hydrogel-based dressing to protect and soothe C-section incision sites.
- The company turned to the team at Scapa Healthcare for its hydrogel expertise.
- Scapa Healthcare created a complete turnkey solution, speeding the time to market delivery of a high-quality product.
Challenge
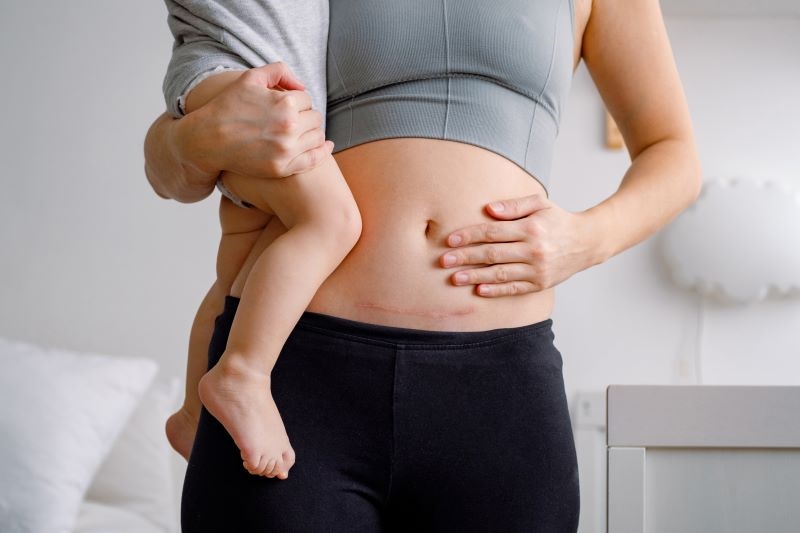
The customer wanted to grow its postpartum care product line by developing a new hydrogel-based dressing to protect the incision site of C-sections. The low-profile dressing prevents clothes from rubbing and soothes the incision site as it heals. However, they had an extremely challenging target launch date, making it difficult to handle new product development in-house. They sought a reliable partner to handle the entire development and manufacturing process, including regulatory support, sterilization, and packaging, to produce a high-quality product to be introduced to consumers via retail channels.
Solution
The customer turned to the Scapa Healthcare Hydrogel Technology Center in Ramsbury, U.K. due to its experience in hydrogel skin-friendly adhesives to provide a complete turnkey solution.
Scapa Healthcare offered the customer a dressing design and hydrogel formulation from within its technology portfolio. This approach saved development costs and significantly reduced time to market. Additionally, the team designed an adhesive and PU film release liner that ensured smooth application and proper adhesion for the recommended wear time.
To expedite the testing process, the Scapa Healthcare team created a branded digital concept to give the customer a visual representation of the product. Within a week, they also developed prototypes for initial research, focus groups, and user feedback. This quick turnaround further accelerated the design process, saving additional months of development time. The Consumer studies conducted by the customer demonstrated that Scapa Healthcare's hydrogel technology outperformed alternative offerings, scoring significantly higher in all categories.
As the product was required to be sterile, Scapa Healthcare validated it using their in-house gamma radiation plant in Gargrave, UK. The customer also was looking for packaging options that were shelf-ready. This process was particularly challenging due to the product's height and space limitations on the retail shelf. Scapa Healthcare worked closely with packaging partners and design teams to produce cartons that met the client's requirements.
The Scapa team also worked closely with the customer to keep them updated on the progress of the validation process, including operational qualification (OQ) and performance qualification (PQ). In addition, Scapa Healthcare's operations and technical teams introduced improvements to expedite production and enhance overall efficiency. Finally, to ensure a successful product launch in both the United States and Europe, Scapa Healthcare provided regulatory support and prepared documentation to meet regulatory standards.
Results
- Scapa swiftly translated initial discussions into a shelf-ready product.
- Through Scapa Healthcare’s collaborative efforts, the customer successfully introduced a new post-birth product to the market at a time of strong demand.
- This allowed them to capture a growing market and strengthen their brand presence.
Scapa Healthcare’s hydrogel expertise and efficient production capabilities successfully fulfilled the customer's needs, ensuring the dressing was ready for distribution by its target launch date. The customer appreciated how Scapa Healthcare’s agile team prioritized effective communication, competitive pricing, and flexible development options, which helped ensure their satisfaction throughout the process.
LOOKING FORWARD
The customer recognized the immense value of partnering with a team that can rapidly progress from concept to production while ensuring compliance with regulatory standards and proving product viability. This positive experience has solidified the customer's trust in Scapa Healthcare as a reliable partner for future product development needs.
ABOUT SCAPA HEALTHCARE
Scapa Healthcare is the trusted strategic partner of choice for the world’s leading companies in advanced wound care, consumer wellness, and medical device fixation. Our deep understanding of the markets we serve allows us to leverage our manufacturing, technology, and development expertise to deliver innovative skin contact solutions that help our customers succeed in the marketplace. Scapa Healthcare offers Regulatory Services for Medical Devices.
