- 9 Sep, 2020
- /
- Category:
- Medical Device Fixation
Developing Skin Safe Pulse Oximetry Devices for Neonatal Care
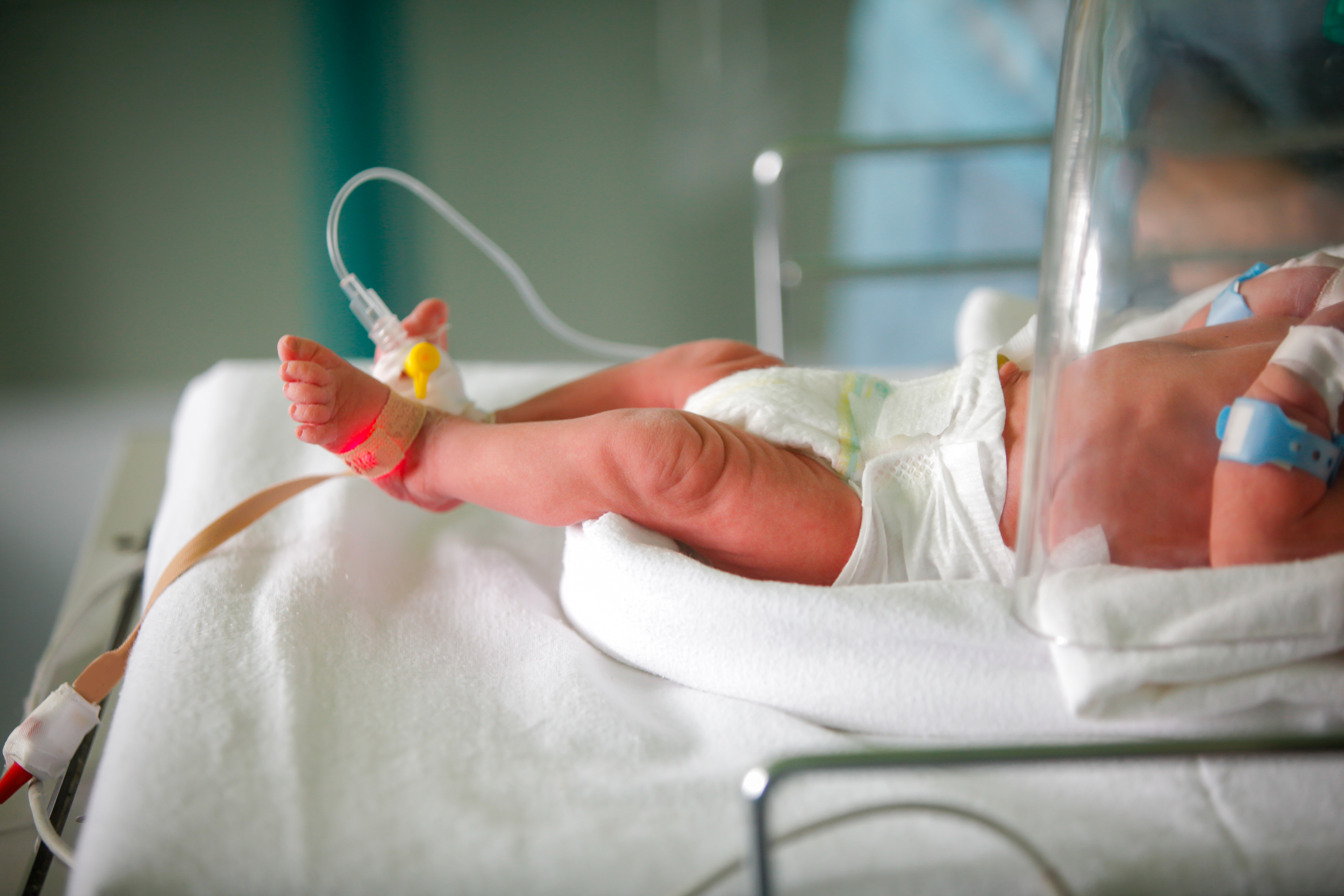
Pulse oximetry involves checking how well the heart is pumping oxygen through the body by attaching a painless sensor to rapidly detect changes in how efficiently oxygen is being carried to the extremities furthest from the heart. Pulse oximetry is particularly important for newborns because during the first few minutes of life, SpO2 (saturation by pulse oximetry) should increase from intrapartum levels of 30–40% to in excess of 90%. But one in every 100 babies is born with a congenital heart defect (CHD) and 25% of those babies will have a critical congenital heart defect (CCHD). Early detection of low oxygen levels and, often, surgical intervention, are key to saving babies with CCHD.1 Given its life saving significance, The American Academy of Pediatrics implemented mandatory use of pulse oximetry in screening newborns.
There are many different pulse oximeters available on the market that are easy to use and safe for babies. When conducting pulse oximetry screening on infants in the delivery room, a sensor is affixed around the whole hand or foot given their small size rather than using a clip-like sensor on a finger or toe that is suitable for adults. Hook-and-loop fastener is the primary means to affix a sensor on a baby but thanks to more recent innovations there are now many skin-safe adhesive options. Adhesives allow the sensor to be placed closer to the body, securely, potentially allowing for a more accurate reading.Pulse oximetry screening is done after 24 hours from birth in order for the baby's heart and lungs to fully adjust to life outside the womb first. The sensor strip is placed on a baby’s skin for a few minutes and then a number is sent to the monitor that gives the doctor information about the amount of oxygen in the baby’s body. The test usually takes about five minutes, but can take longer if the baby is squirming or crying.2
When developing a pulse oximetry device for infants, it is important to choose a low-trauma adhesive such as a silicone gel that can be affixed directly to fragile neonatal skin and repositioned or removed as needed without causing harm or leaving residue. A soft, flexible tear-resistant backing will be used on top of the adhesive and should have elasticity and be able to conform comfortably to the body and provide a secure fit. Pulse oximeters with low-trauma adhesive tapes can also be used on populations with sensitive skin such as geriatrics and patients in intensive care units (ICU).
Scapa Healthcare has the adhesive formulation capabilities and substrate and product development expertise to partner with pulse oximetry device OEMs to create adhesive-based products suitable for device fixation for neonatal care. Scapa also offers value-added services such as printing, which can be applied to creating both child-friendly designs and/or functional printing to aid with registration and assembly of a device. Learn more today.
__________________________________________________________________________________________________________________
1 healthychildren.org, Newborn Pulse Oximetry Screening to Detect Critical Congenital Heart Disease https://www.healthychildren.org/English/ages-stages/baby/Pages/Newborn-Pulse-Oximetry-Screening-to-Detect-Critical-Congenital-Heart-Disease.aspx
2 Children's Minnesota, Newborn Pulse Oximetry Screening https://www.childrensmn.org/educationmaterials/childrensmn/article/16080/newborn-pulse-oximetry-screening/
