literature
Scapa Healthcare is the trusted strategic partner of choice for the world’s leading companies in advanced wound care, consumer wellness and medical device fixation. Our deep understanding of the markets we serve allows us to leverage our manufacturing, technology and development expertise to deliver innovative skin contact solutions that help our customers succeed in the marketplace.
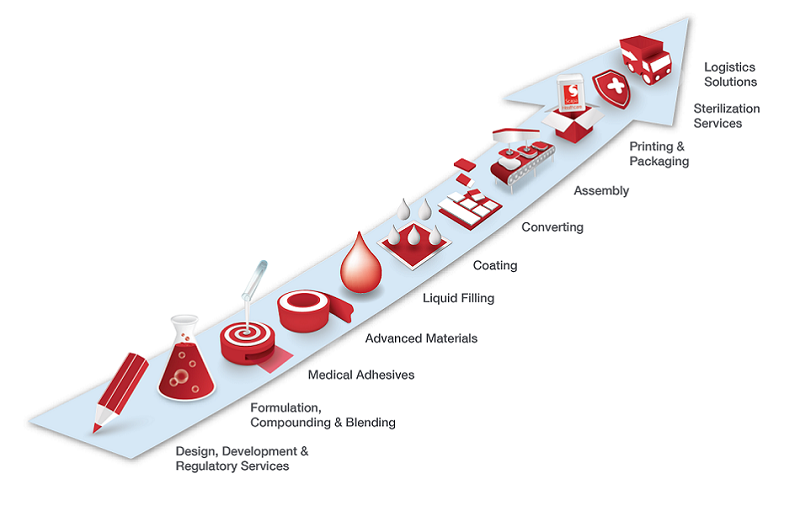
Our Strategy
We collaborate with healthcare market leaders to develop and manufacture innovative skin friendly medical device fixation and skin care topical solutions to improve people’s lives. Through these collaborations, we provide integrated services to the top global medtech companies. Our highly qualified team of experts and state-of-the-art facilities enable us to offer customers the whole spectrum of development and production services from inception through to market delivery. Our ability to deliver differentiated solutions to market faster gives our customers a sustainable competitive advantage in the marketplace.
